Main production process of potassium chloride and potassium sulfate

What is the Production process of potassium chloride?
1. Select potassium salt
Potash salt is generally a mixture of potassium chloride and sodium chloride. The main methods are flotation, dissolution crystallization and heavy medium beneficiation. To produce potassium chloride from potash salt ore, the ore must be crushed first to dissociate potassium chloride and sodium chloride into monomers. The degree of dissociation varies according to the mineralization conditions and production methods. Different production methods require different crushing particle sizes. The flotation method requires a particle size of less than 2.4 mm, while the dissolution crystallization method has a looser particle size requirement.
Flotation method:
A method of producing potassium chloride from potassium-containing slurry using flotation agents. Based on the fact that the surfaces of potassium chloride and sodium chloride crystals are wetted by water to varying degrees, when flotation agents are added, their surface properties can be changed and their surface wettability differences can be enlarged. When air is blown in, small bubbles are generated. Potassium chloride crystals adhere to the small bubbles to form foam and rise to the surface of the slurry. Then it is sieved out in the flotation tank, filtered, washed and dried to obtain potassium chloride products. Sodium chloride is hydrophilic and remains in the slurry and is discharged as tailings. The flotation agents used include:
- Collectors, fatty amines containing 16 to 18 carbon atoms.
- Regulators, which regulate the effects of collectors and frothers and improve flotation conditions. There are generally three types: inhibitors, such as starch, aluminum sulfate, etc.; activators, such as lead salts, bismuth salts, etc.; adjusters, such as sodium carbonate, sodium sulfate, etc.
- Frothing agents, pine oil and monoatomic and diatomic alcohols of the dioxane and pyran series. The fuel consumption of flotation is much lower than that of dissolution crystallization.
Dissolution crystallization method:
This method uses the difference in solubility of potassium chloride and sodium chloride in potash ore at different temperatures to separate them. The potassium chloride mother liquor (brine) heated to 100-110℃ is used to dissolve the potash ore, in which potassium chloride is transferred into the solution, and sodium chloride and other insoluble substances remain in the insoluble residue. The residue is separated by centrifugation, and the clarified liquid is cooled to obtain potassium chloride crystals. The product obtained by this method is of good quality and has strong adaptability to the ore. It is suitable for potassium chloride crystal monomer separation with small particles and relatively complex components of potash ore, but the energy consumption is relatively large. (The process flow is shown in the figure below)
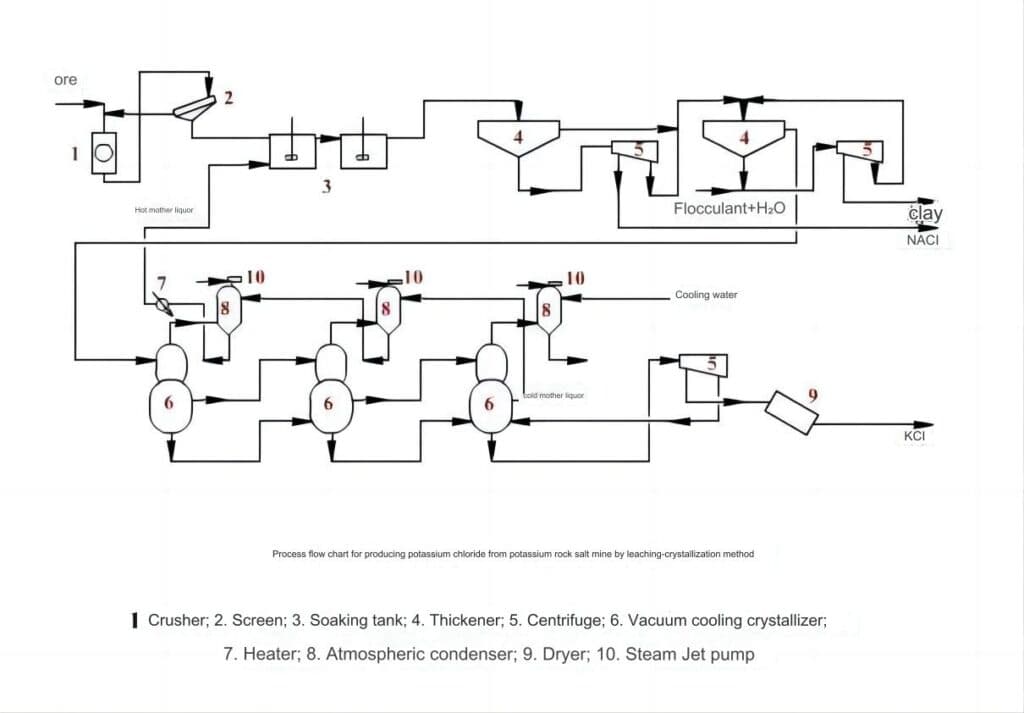
The crushed potash salt is sent to the dissolution tank 3, where it is leached with the hot mother liquor after crystallization. The leached slurry is dehydrated by a centrifuge after sedimentation, and the filtrate and the overflow liquid of the sedimentation tank are sent to the second sedimentation tank for further clarification. The clarified liquid is sent to the vacuum crystallizer to obtain potassium chloride. Generally, multiple crystallizers are used in cascade operation, and the solution flows through each crystallizer one by one. The evaporator is placed in a vacuum by steam injection in the ejector. The vacuum degree increases step by step according to the process sequence. In the last crystallization tank, the solution is close to room temperature. The potassium chloride slurry is then dehydrated by a centrifuge and dried by a dryer to obtain the finished product. The advantages of this method are high potassium yield, less potassium chloride carried away by waste slag, large and uniform crystal particles of the finished product, and high purity. The disadvantages are that it consumes fuel, the leaching temperature is high, and the equipment is seriously corroded.
Heavy medium separation method:
The method uses the difference in relative density between potash salt and rock salt in potash salt ore to separate them by adding a medium with a density between potash salt and rock salt. The heavy medium suspension can be prepared with pyrite powder (or ferrosilicon powder) and saturated brine. This method is suitable for the separation of large-particle, high-grade potash salt ore. If the particle size is less than 1mm, this method cannot be used because the internal adhesion leads to non-selective agglomeration between particles.
2. Select carnallite
The chemical formula is KMgCl3·6H2O, which is a halide mineral of the orthorhombic (orthorhombic) crystal system. It is a mineral raw material for extracting metallic potassium and magnesium, manufacturing potassium compounds, and potash fertilizers. The main methods are complete dissolution method and cold decomposition method.
Complete dissolution method:
Use brine saturated with sodium chloride heated to 105°C to dissolve carnallite. After separating the sodium chloride and insoluble matter, cool the resulting clarified liquid to 25°C to precipitate potassium chloride crystals, which are then washed and dried to obtain the product. The mother liquor is evaporated and concentrated, and after potassium chloride is recovered, a portion is discharged and a portion is returned to the carnallite ore for leaching. The product obtained by this method is of good quality, but the energy consumption is high.
Cold decomposition method:
Use brine or water to dissolve carnallite at room temperature to obtain potassium chloride with very small particle size. Use gravity or centrifugal force to separate the potassium chloride, and then wash and dry to obtain a product with a potassium chloride content of 90% and a fineness of less than 200 mesh. This method is simple to operate, has low energy consumption, and operates at room temperature, which makes the equipment less corrosive. The equipment material can be ordinary carbon steel. The disadvantage is that the product purity and potassium yield are both low, and the product particles are fine.
summary:
Potassium chloride has a wide range of uses. In agriculture, it can be used as potash fertilizer; edible and medicinal potassium chloride is made by adding industrial-grade potassium chloride to distilled water to dissolve into a saturated solution, adding a decolorizer, an arsenic remover and a heavy metal remover to purify the solution, and then precipitating, filtering, cooling and crystallizing, centrifuging, and drying. It is used as a nutritional supplement, a gelling agent, a yeast food, and a salt substitute. Like table salt, it can be used as a flavoring agent for agricultural products, aquatic products, livestock products, fermentation, seasoning, canned food, convenience foods, etc. to make low-sodium products. It is also used to strengthen potassium for human electrolytes and prepare athlete drinks. It is used as a diuretic and a drug for preventing and treating potassium deficiency in medicine. It is used to make potassium salts such as potassium carbonate, produce G salts and active dyes. It is used in photography, electroplating, and steel heat treatment. It can also be used as a flame extinguisher in the military.
Production of potassium sulfate
Potassium sulfate is produced by Mannheim furnace method, double decomposition method, placement method and alum stone comprehensive utilization.
1. Mannheim furnace method
This method is the most mature production method for producing potassium sulfate. The main equipment is the Mannheim furnace (sulfate mechanical furnace). Its production principle is: potassium chloride and concentrated sulfuric acid react in the Mannheim furnace to produce potassium sulfate and liquid hydrochloric acid as a by-product. KCl+H2SO4 K2SO4+2HCl. The reaction is carried out in two steps. The first step is to decompose potassium chloride with sulfuric acid at a lower temperature to produce potassium hydrogen sulfate. The second step is to react potassium hydrogen sulfate with potassium chloride at a higher temperature to produce potassium sulfate. The heat required for the reaction is supplied by heavy oil or gas fuel burning in the combustion chamber of the Mannheim furnace. The combustion chamber temperature is 900 to 1000 degrees, and the reaction chamber temperature is controlled at 520 to 540. The product temperature coming out of the furnace reaction chamber is relatively high and must be cooled.
2. Double decomposition method
The basic principle is to use potassium chloride and the raw materials used to perform double decomposition reaction to produce complex salts with lower solubility, and obtain potassium sulfate and by-products. The double decomposition method does not require the treatment of highly corrosive materials at high temperatures, nor does it require any auxiliary materials. The process and equipment are extremely simple, and the investment is lower than that of the Mannheim method and the dissolution method of the same scale. In addition, it can utilize various industrial wastes, such as ammonium sulfate, mirabilite, magnesium sulfate, etc.
3. Dissolution method
This method is a new process for the production of potassium sulfate developed by my country. The principle of this process is to use the different properties of organic solvents to hydrochloric acid and sulfuric acid to produce potassium sulfate. Sulfuric acid, potassium chloride, and liquid ammonia are used as raw materials, and an organic solvent insoluble in water is used as an associating agent. It is combined with sulfuric acid, and the chloride ions in the solution replace the sulfate ions on the associating agent to obtain a potassium sulfate solution. Then, ammonia is used for decomposition, so that the chloride ions on the associating agent react with the ammonium ions to form ammonium chloride. They are cooled and separated to obtain potassium sulfate and ammonium chloride products, and the associating agent is recycled.
4. Comprehensive utilization of alum stone
The chemical formula of alum stone is KAl3(SO4)2(OH)6. To produce potassium sulfate from alum stone, the alum stone must be crushed, then roasted to remove the crystal water, and then potassium sulfate products are produced using the potassium alum method, reduction pyrolysis method, and ammonia-soda method. The potassium alum method is to first roast the alum stone at 600 degrees to dehydrate it, and then use the dilute sulfuric acid containing potassium sulfate obtained in the process to countercurrently leaching it. When the clarified alum stone solution is cooled to 20 degrees, about 80% of the crystals will precipitate. When it is difficult to filter, the slurry can be crystallized together, and then the crystals with mud separated from the mother liquor are dissolved and filtered under pressure to obtain a clarified alum solution. In an acid-resistant autoclave, alum is hydrolyzed under pressure at a temperature above 150 degrees to convert aluminum oxide into basic alum, and dilute sulfuric acid is generated for dissolving and dehydrating alum stone. The basic alum is then calcined at a high temperature of about 1000 degrees to generate non-absorbent aluminum oxide. Potassium sulfate can be crystallized from the solution after cooling.
Conclusion:
Potassium sulfate has a wide range of uses and is often used as medicine (such as laxatives) and fertilizer (containing about 50% potassium, it is a fast-acting potassium fertilizer that can be used as base fertilizer, seed fertilizer and topdressing). It is also used to make alum, glass and potassium carbonate, etc. It is used as a chemical fertilizer in agriculture. It is a raw material for making potassium salt. It is used in the dye industry to make intermediates. It is used as a clarifier in the glass industry. It is used as an auxiliary agent in the spice industry. It is used as a laxative in medicine. It is used as a general additive in the food industry. It is also used for biochemical testing of serum proteins. It can also be used as medicine, potassium fertilizer, and used to make alum, glass and potassium carbonate, etc. It can be used as a dietary salt substitute.